Ceftriaxone Sodium
Ceftriaxone sodium
CAS №: 74578-69-1
EINECS №: 277-930-0
MF: C18H18N8O7S3
MW: 554.58
Structural Formula of Ceftriaxone Sodium
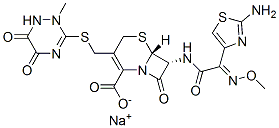
Specifications of Ceftriaxone Sodium
Physico-chemical Properties: White to yellowish crystal powder
Density: / g/ml
Boiling point: / ºC
Melting point: / ºC
Flashing point: / ºC
Product specification: / ug/mg
Main Uses: API
Packaging: 5KG net in a cardboard drum with inner aluminum foil bag
What Is Ceftriaxone Sodium Injection Used For?
The antibacterial spectrum of ceftriaxone sodium was similar to that of cefotaxime sodium. It has strong effects on Escherichia coli, pneumobacillus, Indole positive Proteus, Influenzae, Serratia, Meningococcus, and Neisseria gonorrhoeae. Pneumococcus, Streptococcus, and Staphylococcus aureus were moderately sensitive to ceftriaxone sodium. It has a certain effect on Pseudomonas aeruginosa Enterococcus, methicillin-resistant Staphylococcus, and most of the Bacteroidetes fragilis are resistant to the ceftriaxone sodium.
At present, the ceftriaxone sodium injection is widely used for the treatment of pneumonia, bronchitis, peritonitis, pleurisy caused by sensitive bacteria. You can also find the ceftriaxone sodium injection uses for the infection of the skin and soft tissue, urinary tract, biliary tract, bone and joint, facial features, wound surface, and other sites.
What Are The Side Effects Of Ceftriaxone?
Allergic reactions of ceftriaxone sodium can cause rashes, fever, itching, etc. Sometimes, ceftriaxone sodium can also lead to a lack of appetite, nausea, vomiting, and diarrhea appear in the digestive system. Occasionally, leukocytes, neutrophils, thrombocytopenia, and eosinophilia can also be observed. Long-term use of ceftriaxone sodium sterile can cause double infection, such as candidiasis, pseudomembranous enteritis, etc. Thus, before using ceftriaxone sodium injection, it is suggested to check whether the patient has allergic reaction towards the ceftriaxone sodium or not.
+86-531-69959201
sales@saigaonutri.com
No 12111,Jingshi Road, Lixia District, Jinan City, Shandong Province. P.R. China




